What does it look like?
Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
What is it?
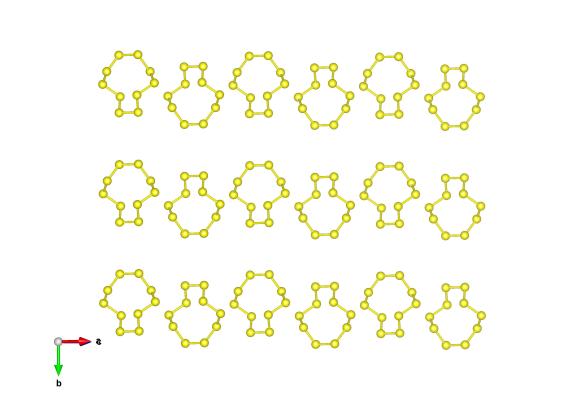
When engulfed in fire and brimstone, have you ever wondered what brimstone actually is? Well in this context it’s referring to sulfur – with all the negative connotations that this yellow, smelly substance has. We’ve already featured one allotropes of sulfur, the S10 rings. But as we explained back then sulfur is probably the element with the most different ways of packing itself in a crystal structure – many have been discovered so far!
Many of the sulfur structures are composed of rings of sulfur atoms – today’s is no exception to this. Instead of rings of 10 sulfur atoms, here we have the more common form rings of 8 atoms.
Where did the structure come from?
This structure we’ve featured today is that of alpha-sulfur, which was first reported by Coppens et al, we obtained the CIF (which is hosted on the Inorganic crystal structure database) through the chemical data service (CDS) links on this page.