What does it look like?
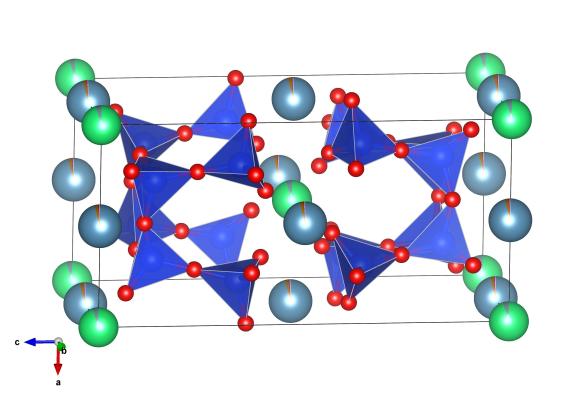
The crystal structure of Ekanite. The green atoms are the thorium which supply the radioactivity that breaks down the crystal structure over time. Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
What is it?
Ekanite is a very rare gem, found originally in Sri Lanka, that is a olive green/brown colour. Not only is it quite a rare gem, but it also does something very unusual – it destroys it’s own crystal structure. This is because ekanite contains thorium, an element that often has high levels of radioactivity. The energy from this is enough to break down the crystal structure, and turn the material into an amorphous material, a glass where the atoms are arranged with little long range order. This phenomenon is known as metamictization, and does actually occur in another mineral we’ve featured – Zircon – when the inclusions of thorium are high enough.
Where did the structure come from?
The crystal structure of ekanite is #9004161 in the Crystallographic Open Database




