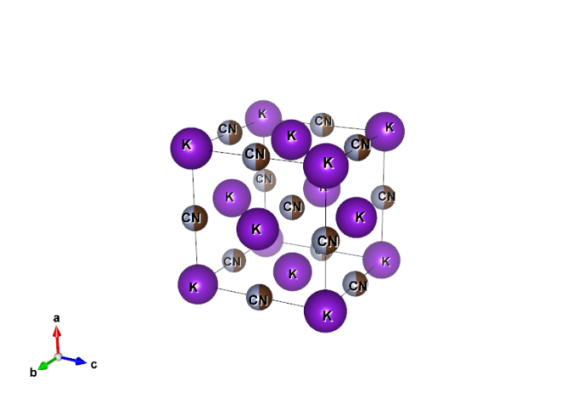
What does it look like?
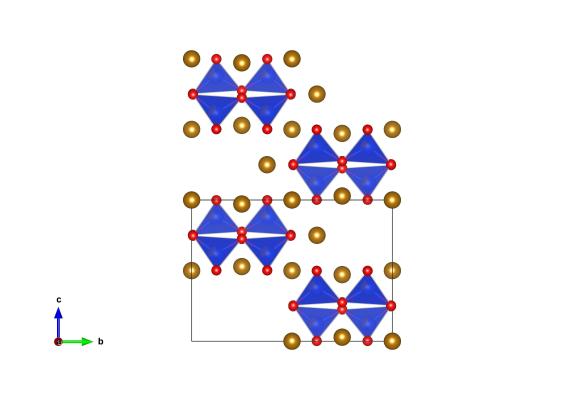
Image generated by the VESTA (Visualisation for Electronic and STructual analysis) software http://jp-minerals.org/vesta/en/
What is it?
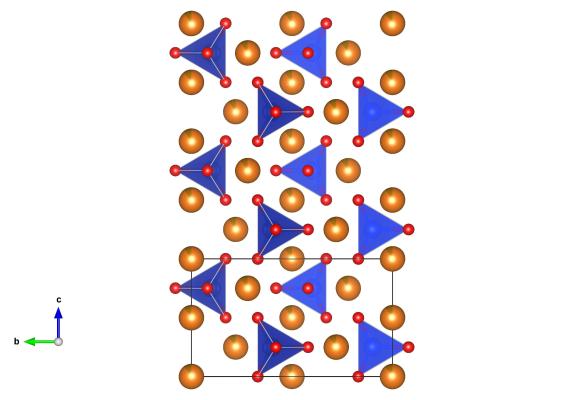
As you delve deeper into our planet not only does the heat start increasing but the burden of the weight of rocks above means that pressures start rising too. At approximately 410 km under our feet these pressures and temperatures are enough to change the structure of olivine to sometime new. What happens is that the silica tetrahedral pair up to S2O7 groups, and the magnesium atoms are spaced between these. Though, on Earth, it is thought to exist only within the mantle it has been found in a meteorite on the surface. It’s thought that in this case wadsleyite could have been formed by shock pressures from impacts out in space.
Where did the structure come from?
The structure of wadsleyite, from a crystal studied while it was under 9 GPa of pressure is #9002372 in the open crystallographic database.